Decentralized trials and their impact on enrollment
Introduction
The COVID-19 pandemic has had a transformative impact on the delivery of clinical trials. The implementation of social distancing, quarantines, and stay-at-home orders has resulted in unprecedented delays and disruptions to operations across the globe, and thousands of trials have been suspended or stopped in 2020. The pandemic has also served as an accelerant for the adoption of technology-based innovations that have steadily been gaining traction for years. Perhaps the most significant evolution in clinical trial design and delivery throughout 2020 and into 2021 has been the wide-spread adoption of technologies to facilitate decentralization of clinical trials via solutions like digital recruitment, virtual visits and eCOA captured via telehealth.
According to the Society for Clinical Data Management (SCDM), one of the primary risk areas associated with clinical trials has been the dependency on research staff being continuously and physically on-site. Simple deviations to staff schedules, such as challenges to daily commute or personal leave, can cause delays to data entry, and to site initiations and enrollments. In 2020, COVID-19 has transformed these constant background risks into pressing global issues. Several studies have sought to understand the broad impact of COVID-19 on clinical trials, but few have shown how this impact has differed between traditional site-based trials and those employing decentralized approaches for trial data collection. Now, more than a year after initial quarantines and stay-at-home orders took effect, we are able to start to understand whether the application of decentralized methods has allowed certain clinical trials to overcome these challenges.
The analysis described is not intended to be a comprehensive assessment of the impact of decentralized approaches on clinical trial success. The authors selected two (2) trial performance metrics pertinent to clinical trial success and conducted the analysis with the goal of providing relevant information for industry, as research sponsors look to integrate lessons from 2020 into future clinical trial designs. Additional analyses will be conducted in the future to supplement the analysis described below to more comprehensively and holistically address how decentralized trials fared in other performance focus areas against traditionally designed trials in 2020.
Scope of Analysis and Methodology
To better understand the impact of decentralized approaches on clinical trial success during pandemic-driven shutdowns, THREAD and Lokavant conducted an analysis to understand how clinical trials delivered on the THREAD DCT Platform, and traditional clinical trials with traditional site-based patient visits, adapted to the pandemic over the first three quarters of 2020. This time period allowed for observation of pre-COVID performance in January and February, immediate response to widespread quarantine and shelter-in-place orders in March and April, and subsequent peri-COVID performance through September.
For the initial analysis, THREAD provided operational performance data from ongoing decentralized trials within the selected time window and two (2) metrics corresponding with available THREAD operational performance data were selected from Insight, Lokavant’s clinical operations benchmarking solution: Subject Enrollment Rate and Subject Discontinuation Rate. The relevant Industry Benchmark, taking into account key parameters of the THREAD Data Set, was calculated utilizing Insight, which draws on comprehensive operational data on over 1,300 historical trials and near real-time data on on-going studies. To avoid any complications with direct comparison of absolute values across THREAD and Industry trial datasets, respective datasets were min/max normalized and results are shown relative to respective January reference points.
Both Data Sets comprise a mix of study phases, therapeutic areas, and geographies. The THREAD Data Set includes hybrid and fully decentralized trails, and one registry study.
Results
Subject Enrollment
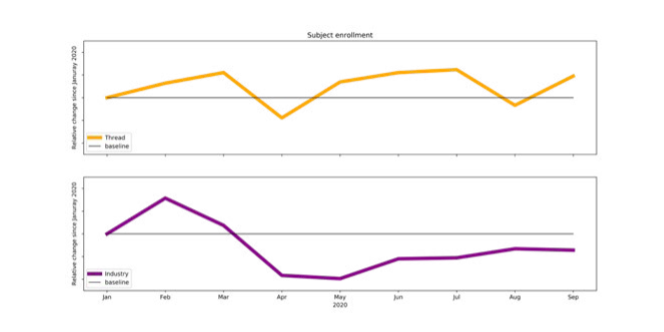
The analysis of subject recruitment demonstrates that, while trials in the THREAD Data Set observed a decline in enrollment relative to a January baseline in March, at the onset of widespread quarantine and shelter-in-place orders. Enrollment then rebounded between April and May. Additionally, recruitment for these trials continued to exceed the pre-COVID baseline value in five out of the six months from May to September. The Lokavant Industry Benchmark observed an earlier and steeper decline in subject recruitment from February to April. Enrollment improved from April to June but stabilized below its corresponding pre-COVID baseline value.
Subject Discontinuation
The analysis of subject discontinuation similarly indicates that dropout rates for both decentralized trials and traditional site-based trials were significantly impacted at the onset of global quarantine and shelter-in-place orders. However, trials in the THREAD Data Set demonstrated a return to pre-lockdown subject discontinuation by June of 2020 while the Industry Benchmark presents a continuing rise in discontinuation through the end of September 2020.
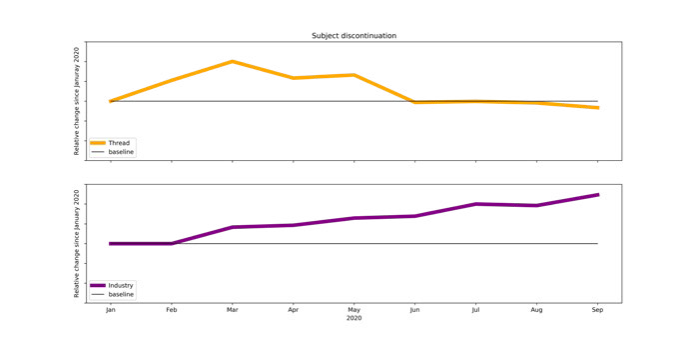 The analyses presented indicate that decentralized clinical trials (DCTs) were able to return to their pre-COVID enrollment rates and discontinuation rates within 2-4 months of widespread global lockdowns in February and March of 2020. The analyses also indicate that traditionally designed clinical trials were both unable to return to pre-COVID enrollment rates and were continuing to see increasing discontinuation rates more than 6 months after lockdown measures came into effect. The THREAD Data Set contained studies using a range of decentralized approaches (from hybrid to fully decentralized), so how can we begin to explain how a decentralized design had such a profound impact on patient recruitment and retention?
The analyses presented indicate that decentralized clinical trials (DCTs) were able to return to their pre-COVID enrollment rates and discontinuation rates within 2-4 months of widespread global lockdowns in February and March of 2020. The analyses also indicate that traditionally designed clinical trials were both unable to return to pre-COVID enrollment rates and were continuing to see increasing discontinuation rates more than 6 months after lockdown measures came into effect. The THREAD Data Set contained studies using a range of decentralized approaches (from hybrid to fully decentralized), so how can we begin to explain how a decentralized design had such a profound impact on patient recruitment and retention?
The Value of Decentralized Approaches
Virtual visits cannot replace in-person site visits for every study, but they can be used in place of some in-person visits (a hybrid decentralized study) and create a more flexible and patient-centric model to drive improved patient engagement. Some tangible ways virtual visits and remote patient monitoring technologies can help and have helped sponsors, CROs and clinical trial suppliers keep clinical trials moving forward both during and beyond the pandemic are:
Recruit candidates without needing to visit a site. Qualifying participants is an activity that can take place with home health assessments and a telehealth call to capture relevant information.
Minimize or eliminate travel for participants. Getting to and from frequent research site visits can be challenging, even under normal circumstances. Offering virtual visits and risk-based quality management is even more essential in a pandemic environment as it allows the study to continue even under strict quarantine requirements.
Maintain enthusiasm and trial momentum even amidst strict lockdowns. Telehealth virtual visits enable those conducting the study to easily communicate and share information with participants, stay in contact with patients and provide an opportunity to collect study data during a virtual visit from eCOA, CRFs, sensors, eDiaries and other assessments.
Strengthen relationships between researchers and patients. Trust-based relationships can take a long time to build, especially if contact is limited because of lockdown requirements. Telehealth enables those patient visits to continue with multiple short encounters, helping create a stronger personal relationship that drives retention.
Ultimately, additional analyses need to be performed to understand how each of these value drivers impacted recruitment and retention outcomes for the decentralized trials in the THREAD Data Set, but perhaps the most important factor is that each of the trials had been designed with some form of decentralized technology embedded in the study. As global lockdowns started to come into effect in the early part of 2020, researchers running decentralized trials were able to increase the amount of decentralization in their study, allowing these studies to continue to experience the benefits described above. For researchers overseeing traditionally designed trials, implementing decentralized technologies was much more challenging because the protocol had not been designed with these technologies in mind; as a result, they were not in a position to effectively pivot.
Conclusions & Recommendations for Industry
The maturation of decentralized clinical trial features like telehealth, eConsent and eCOA means that going forward, technology-based solutions can and should be deployed in clinical trials routinely to allow for maximum operational flexibility.