Published on:
June 17, 2022, 4:00 AM EDT
There are countless myths surrounding the clinical research industry, which, unfortunately, often inform critical decision-making in clinical operations and development. As part of a new blog series, we will conduct retrospective analyses using Lokavant’s deep data compendium, leveraging leading data science approaches to assess the veracity of these myths, once and for all. This blog post focuses on the clinical operations myth that 20-percent of sites fail to enroll a single patient into a trial. This myth has been relied upon by sponsors and CROs for decades, during critical feasibility processes, as well as when reforecasting study enrollment. But is it true? Let’s find out!
Based on our analysis of approximately 7,500 sites (a subset of the Lokavant data compendium), with data from completed interventional studies from 2010 to 2020, we found that the reality is much more complex and troubling for the state of enrollment. We found that only 17-percent of sites conducting studies fail to enroll a single patient, but 42-percent of this subset failed to even screen a patient. This is alarming since sponsors invest a lot of resources in supporting site readiness and activation. In addition, another 24-percent of sites conducting multi-center, Phase I through IV, studies only enroll one or two patients.1 In summary, approximately 41% of sites will under enroll.
Phase*
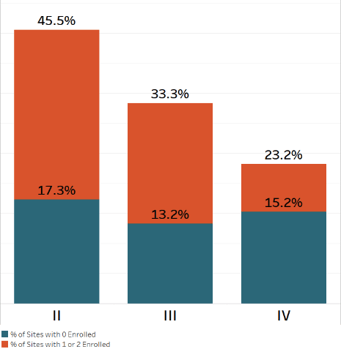
- Almost half of sites in Phase II either don’t enroll or under-enroll (only 1 or 2 patients); the same for one-third of sites in Phase III
- Overall, the 46-percent of sites that failed to enroll also failed to screen a single patient
Therapeutic Area*
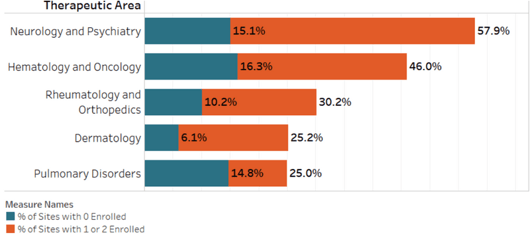
- Dermatology had the lowest number of non-enrolling sites
- Almost one half of sites in Hematology/Oncology trials didn’t enroll
Region*
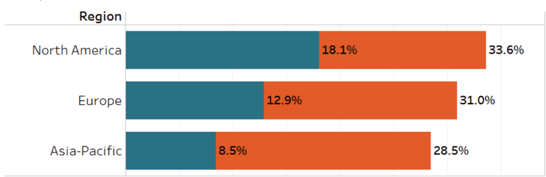
- Site enrollment varies depending on its geographic location
- In Europe, North America, and Asia-Pacific approximately one third of sites are either non-enrolling or under-enrolling
Overcoming Enrollment Challenges
There are multiple challenges to successful enrollment, so how can we, as an industry, overcome them? With Lokavant’s clinical trial intelligence platform, Oversight, and our deep, proprietary data compendium, sponsors and CROs can use predictive analytics to be proactive in: study design and planning, enrollment forecasting, site selection, and overall study risk assessment. Imagine the impact of knowing the trajectory of a study earlier; how much valuable time and money could be saved? Would you rethink an on-site versus virtual visit in the follow-up portion of the study due to a potentially decreasing retention rate? Would you select more sites in a particular region since they appear to excel in a specific therapeutic area? Would you apply more resources on protocol training because you noticed an increased risk in major deviations? These are all questions that Lokavant can help answer.
Industry veteran, Ken Getz, emphasized the importance of a data-driven approach in 2011, “eventually, metrics-based approaches to routinely and consistently identify top performing investigative sites may become more effective.”2 While our results showed an overall improvement in enrollment, it is evident that the industry is still struggling with recruitment and screening, which can only be improved by proactive, not reactive, trial design and management.
Stay tuned for more MythBusters and let us know of any myths you’d like to see “busted”!
* Only including sites with at least three planned randomized patients
1 There is one Phase II study with only one site included in this sample
2 Applied Clinical Trials

