DEI Oversight
Overcome the challenges of planning, operationalizing, and monitoring DEI requirements
DEI Oversight helps study teams meet new DEl regulations from study planning to execution by monitoring for diversity in real-time to meet DEl requirements.
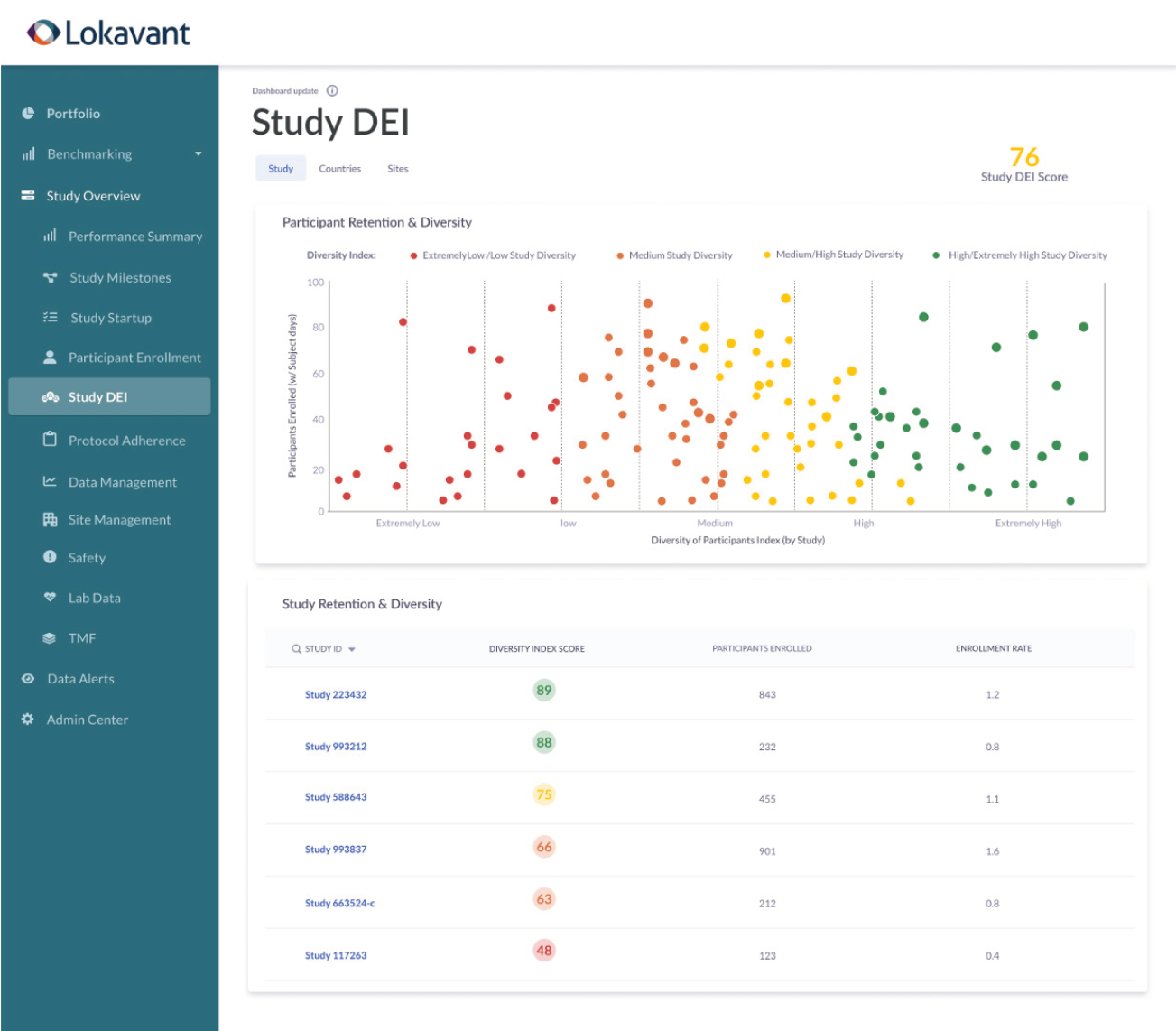
Real-time diversity monitoring
DEI Oversight helps study teams meet new DEI regulations from study planning to execution by monitoring for diversity in real-time to meet DEI requirements.
Site Diversity benchmarking
Leverage prior study data, AI and ML to define DEI thresholds and diversity performance scores to benchmark sites and identify site performance.
Meet new FDA regulation with data-driven DEI planning
Access Lokavant’s data asset to plan and meet regulatory DEI planning requirements and account for underserved populations.
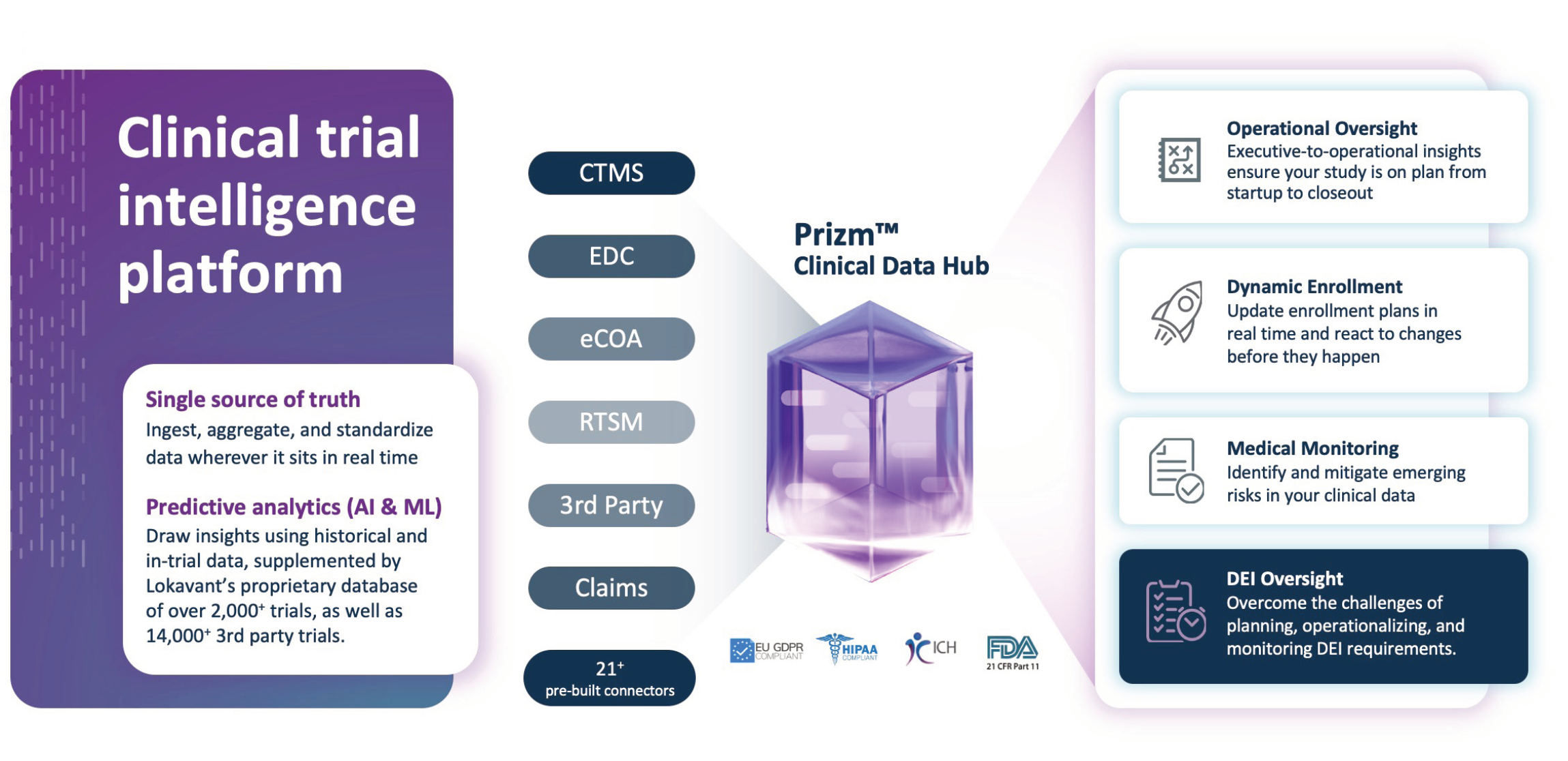
Monitoring and planning diversity and inclusion for compliance in real-time
Three ingredients for DEI in Clinical Trials execution:
- Source agnostic data
- Real-time feedback loop
- Predictive forecasting
Key Benefits
- Incorporate DEI into study planning, site selection & execution
- Identity study performance and patient enrollment to comply with DEI regulations
Plan
Data aggregation
& harmonization
Leverage SDoH and other DEI related data to plan and identify the right sites for diversity. Trial data from source is then ingested and harmonized in real-time.
Operationalize
Central dashboard for
real-time feedback loop
A single, self-service view of real-time study performance with automated reporting and benchmarking.
Monitor
Predictive DEI milestones,
forecasting & risk
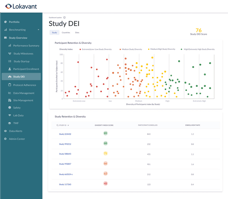
Daily reforecasting of enrollment and site performance is compared against historical performance to provide actionable insights as it relates to DEI.
Features
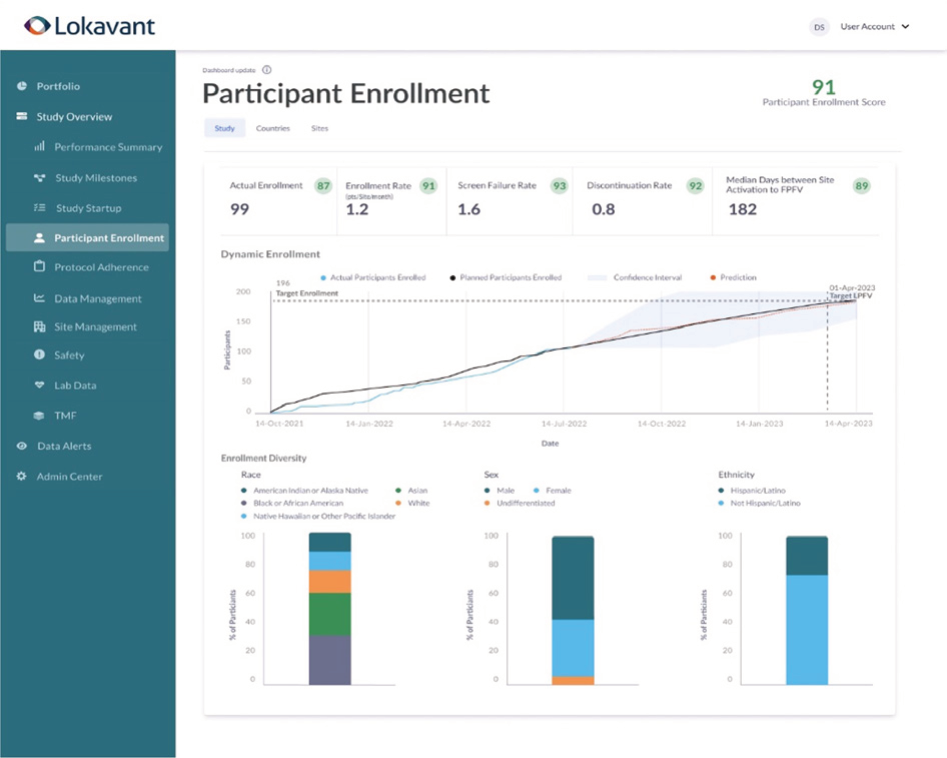
Real-time diversity monitoringOperationalize DEI easily to assess in real-time the diversity mix at a study, country and site level to ensure alignment with your diversity action plan targets
|
 |
Why Lokavant
Lokavant’s Clinical Trial Intelligence platform offers clinical researchers and study teams solutions to predict trial performance. Leveraging our Clinical Data Hub with over 21+ source connectors and proprietary data from over 2000+ harmonized trials and 14,000+ 3rd party trials, Lokavant can help you design, plan and execute trials more efficiently and give you the confidence to make informed decisions.
Featured Resources
Sponsors and CRO’s love us








Get in touch
Schedule a demo to learn how Lokavant can transform your clinical trials.


